Introduction:
Therapy-related myeloid neoplasms (t-MNs) account for about 10 to 20% of all cases of AML and MDS. Allogenic hematopoietic cell transplantation (allo-HCT) is considered as the only curative treatment in this high-risk setting. Given the rise of long-term cancer survivors, an increasing number of patients are expected to become transplant candidates. Physicians are concerned about the frailty and the risk of recurrence of primary cancer. In this retrospective registry-based study, we focused on patients with t-MN secondary to breast cancer radio- and/or chemotherapy, and collected information about the outcomes and complications after allo-HCT.
Methods and results:
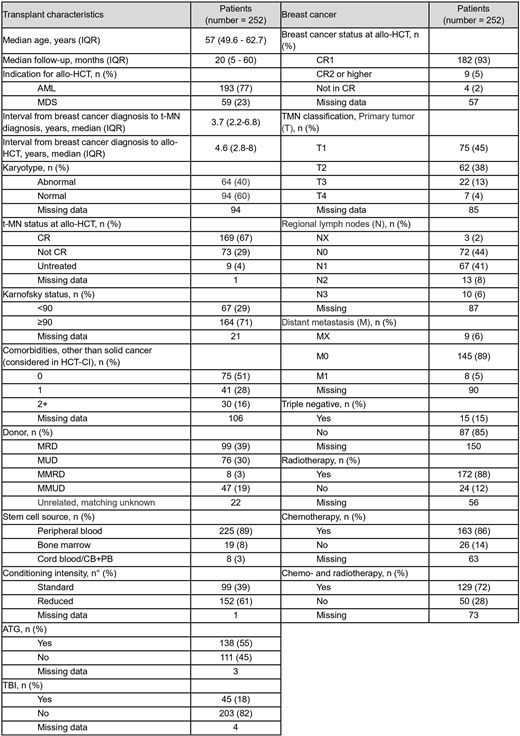
In the EBMT registry we identified 252 female adult patients who underwent an allo-HCT between 2006 and 2016 for t-MN secondary to breast cancer treatment. Median age at transplantation was 57 years, and the median time from the breast cancer diagnosis to t-MN diagnosis and subsequent allo-HCT were 3.7 and 4.6 years, respectively. The indication for allo-HCT was AML and MDS in 77% and 23% of cases, respectively. Sixty-seven% of patients were in complete remission (CR) of their t-MN at the time of transplant. Abnormal karyotype was recorded in 40% of cases. A reduced Karnofsky performance status (KPS<90) was noted in 29% of cases, and 44% of patients presented with at least one comorbidity other than the primary malignancy. Stem cell source was peripheral blood in 89% of cases. Conditioning regimens were reduced-intensity (RIC) in 61% and myeloablative (MAC) in 39% of patients, respectively, and in vivo T-cell depletion was applied in 55% of cases. TBI was used in 18%. Thirty-nine% of donors were matched related (MRD), and 30% matched unrelated (MUD). For the breast cancer, at the time of diagnosis, 5% presented with distant metastasis and 15% were triple negative; 86% received chemotherapy and 88% radiotherapy, 72% both. Of note, at the time of transplant, 93% of patients were deemed in first CR of their breast cancer, and additional 5% were in second CR or more: data on remission status were missing for 57 of patients. Median follow-up from transplant was 20 months and for the entire cohort the 2-year overall survival (OS) was 50% (95%CI 44-56%): in univariate analysis (UVA), an abnormal karyotype, being transplanted not in CR for the hematological malignancy and a worse KPS were associated with a worse prognosis [2-year OS: 41% (28-53%) for abnormal karyotype, 52% (42-62%) for normal, p=.035; 57% (49-64%) for CR, 56% (23-88%) for untreated, 34% (23-45%) for not in CR, p=.002; 55% (47-63%) for KPS≥90, 43% (31-55%) for KPS<90, p=.05]. The 2-year relapse-free-survival (RFS) was 45% (39-52%): as observed for the OS, a detrimental effect on RFS was observed for abnormal karyotype [2-year RFS: 34% (22-46%) for abnormal, 49% (39-59%) for normal karyotype, p=.034] and not being transplanted in remission for the t-MN [52% for CR (44-59%), 44% for untreated (12-77%), 31% (20-42%) for not in CR, p=.002]. 2-year relapse incidence and NRM were 33 (27-39%) and 22% (17-27%), respectively. An abnormal karyotype was significantly associated with a higher relapse rate compared to a normal [50 (37-62%) vs 26% (17-34%), p<0.001], as did an uncontrolled t-MN at the time of transplant [27% (20-34%) for CR, 33% (3-64%) for untreated, 47% (35-59%) for not in CR, p<.001]. Patients pre-exposed to radiotherapy for breast cancer experienced a higher relapse rate compared to those who did not [35 (28-43%) vs 17% (2-33%), p=.04], while the NRM was lower [18 (12-24%) vs 38% (19-58%), p=.02]. Acute grade II-IV GVHD occurred in 26% (22-33%) of patients, and the 2-year incidence of chronic GVHD was 41% (35-47%) including 22% (17-27%) of extensive grade. Seventeen cases of breast cancer recurrence were observed, of which 13 invasive. A curative approach was adopted in 7 cases. The most frequent cause of death in the whole cohort was relapse of t-MN (36%), followed by infection (28%) and GVHD (16%): the relapse of the primary breast cancer accounted for 10 (7%) cases of death.
Conclusions:
Our study shows that allo-HCT can be safely performed in patients with t-MN secondary to treatment to breast cancer. A major drawback is the relapse of the primary disease which nevertheless appeared a rare event. Tight collaboration and multidisciplinary discussion between the oncologist and the hematologist are fundamental, and an adequate follow-up for the solid malignancy is essential thereafter.
Ganser:Novartis: Consultancy; Celgene: Consultancy. Scheid:Takeda: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Amgen: Honoraria; Janssen: Honoraria, Research Funding; BMS: Honoraria. Yakoub-Agha:Celgene: Honoraria; Novartis: Honoraria; Gilead/Kite: Honoraria, Other: travel support; Janssen: Honoraria; Jazz Pharmaceuticals: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal